Hydrogen is a more promiscuous element than chemists have appreciated: it can form up to six strong chemical bonds in some solids, American researchers report.
The finding is of more than academic interest, say Anderson Janotti and Chris Van de Walle, of the University of California, Santa Barbara, US. They suggest that multi-bonding hydrogen atoms are acting as impurities, or dopants, in materials like zinc oxide, increasing their electrical conductivity. Controlling this effect could boost the cause of zinc oxide in future technologies, like solid-state lighting or flat panel display screens.
Hydrogen often associates weakly with other atoms. The open structure of ice, for example, is explained by small but important hydrogen bonding attractions. But when it comes to strong covalent bonding, hydrogen atoms are rather conservative. With just one electron each to share around, a hydrogen atom forms one, two or (at most) three strong chemical bonds with neighbouring atoms in a solid, say Janotti and Van de Walle.
Now the researchers have shown, by quantum mechanical calculations, that a hydrogen atom substituted into a zinc or magnesium oxide lattice bonds strongly – and equally – to four or six metal atoms respectively. This unusually generous arrangement has been termed a multicentre bond. The results are supported by previously unexplained spectroscopic measurements on the solid oxides.
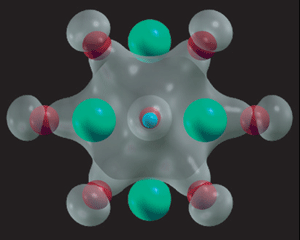
Hydrogen (blue) is bonded to 12 surrounding oxygen atoms (red) in magnesium (aquamarine) oxide
© Nature Materials
|
‘Hydrogen is surely the most widely studied atom, and has often been at the core of new insights in chemistry and physics. But our work shows that hydrogen can still surprise us,’ Van de Walle told Chemistry World. Carlo Gatti, of the University of Milan, Italy, agreed that the results were intriguing, although he wanted to see further confirmation of the suggested bonding.
For zinc oxide especially, the results have immediate practical significance. As Matt McCluskey, of Washington State University, US, explained, zinc oxide may provide a superior solid-state source of lighting, to replace electrical filaments or gas. It is cheap to synthesize, transparent, environmentally friendly, and emits light efficiently with only a small electrical current. But, says Van de Walle, zinc oxide tends to exhibit sporadic and unintentional electron conductivity, confounding potential applications.
The American scientists have now identified hydrogen as a culprit: the multi-bonding atoms act as impure dopants, donating electrons to the material. Once the effect is fully understood, it can be suppressed: perhaps by heating zinc oxide to drive out the hydrogen, suggests McCluskey.
But Van de Walle adds that hydrogen might also be added on purpose to increase the conductivity of zinc oxide; in laptop and TV flat-panel displays, for example, transparent conducting materials are needed to carry current to liquid-crystal pixels.
Richard Van Noorden




